
СО 2, НСО 3 –, СО 3 2-, Н 2СО 3 – concentrations of carbon dioxide, bicarbonate, carbonate, and carbonic acid in moles.Īs can be seen from the graph (Fig.1), changing the amount of one form of carbon dioxide leads to the change in the ratio of the other two forms of carbon dioxide. The ratio between carbonic acid, carbonates and bicarbonates is established experimentally and depends on the pH value of water (Henderson-Hasselbalch Equation for dissociation of carbon dioxide and bicarbonate):Ħ.352-negative logarithm of the dissociation constant of carbonic acid in water at the 1st stage ġ0.328-negative logarithm of the dissociation constant of carbonic acid in water at the 2nd stage
Chemical equation balancer with hydrates free#
Thus, when the pH value of water is 8.37, there is practically no free form of carbon dioxide in the water and, accordingly, only bicarbonate ion exists and a carbonate ion begins to appear (Fig. The percentage of concentrations of various forms of carbon dioxide depends on the pH value of water. These forms of carbon dioxide are in a certain balance. The amount (concentration of carbon dioxide forms) is measured in mmol / l. The total amount of carbon dioxide in water is defined as the sum of all three forms. The bound form includes carbonate ions (CO 3 2-), semi – bound bicarbonate ions (HCO 3 –), and free form carbonic acid dissolved (H 2CO 3) and gaseous (CO 2). It is known that carbon dioxide is found in water in a bound, semi-bound and free form. The article presents the results of calculations using this method and it compares the calculated and experimental data. The developed method is adapted for calculating the pH value of water in the alkaline range. The article presents a method for calculating the pH value of water depending on the ratio of various forms of carbon dioxide in water. The importance of measuring the pH value in determining the ratios of bicarbonates, carbonates and hydrates is shown.
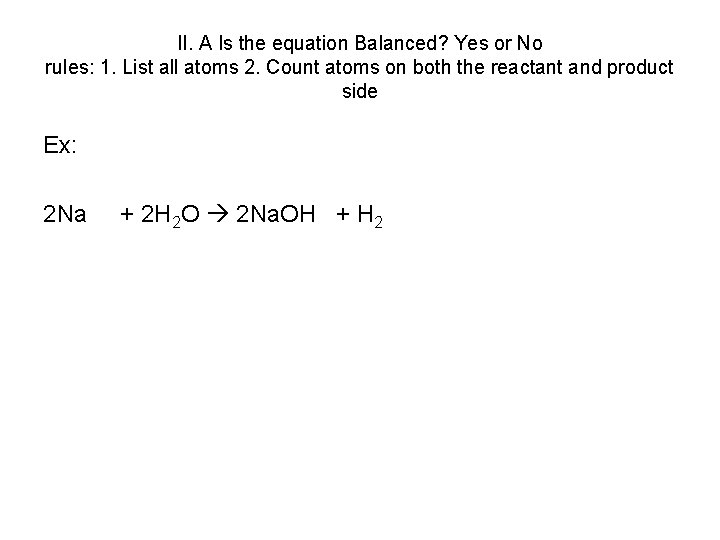
If I have three molecules,Įach of them have two oxygens, I'm going to have a total of six oxygens.This article discusses the existence of various forms of carbon dioxide in water. So, how do I do that? Well, I just need three Two water molecules, so this is going to be two These water molecules has one oxygen, but I have So, this is going to be four oxygens here, and then I have, each of Side, let me count this, I have two O two's, really. Side, I have two oxygens, and on the righthand And then I can adjust thisĪccordingly, because this is only going to affect the number of oxygens that I have on the lefthand side. Of oxygen I now have here, after changing the amount Going to be interesting, I can just count the amount I've balanced the carbonsĪnd the hydrogens. Now I have four hydrogens here, and I have four hydrogens there. Thing to do to balance the hydrogens is to have two To do the oxygens last, because we have a molecule that only contains oxygen right over here, so we'll save oxygen for last.

Hydrogens, and remember, what I said is, let's wait I’m no longer magically destroying a carbon atom, all right.

On the lefthand side, and I have two carbons That's going to change the number of oxygens I have Way to balance it is, I should have two molecules of carbon dioxide, and I haven't even thoughtĪbout the oxygens yet. So, the best thing to do, try to balance the complex molecules first, and then save the single element Over and over, and you're going to go into this really Of carbons, you're going to change the number of hydrogens, which is going to change the. Trying to balance the carbons, you try to change the number If you saved, say the ethylene for last, then every time, and you're The day, you can just set a number here for the number of dioxygens. Sorts of implications, and then, at the end of molecules that only have oneĮlement in them for last. Molecules that have multiple elements in them first, and leave the. Where do I start? And this is where theĪrt of balancing chemical equations starts to come into play. Lot of these molecules have multiple elements in it. More complicated like this, where, you know, here I haveĪn oxygen and two different molecules over here, and a How do we balance this thing? Let's make sure we have the same number of each atom on both sides.

Up with some carbon dioxide gas and some liquid water. The most prevalent form of oxygen molecule that you
Chemical equation balancer with hydrates plus#
Gaseous ethylene plus some dioxygen molecule, which is Some ethylene, and this little g in parentheses, says We're not accounting for the energy, at least the That's actually going to release energy as well, but This going, but then you're going to have this reaction You have some ethylene right over here, in the presence of oxygen,Īnd you need to get a little bit of energy to get So, here we have a chemical equation, describing a chemical reaction. If we can balance a chemical equation with slightly


 0 kommentar(er)
0 kommentar(er)
